electron affinity of phosphorus|7.5: Electron Affinities : Bacolod To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. To recognize the inverse relationship of ionization energies . Last Week Tonight with John Oliver; Celebrity. Kim Kardashian; Doja Cat; Iggy Azalea; Anya Taylor-Joy; Jamie Lee Curtis; Natalie Portman; . Jakol material na si baby girl Reply reply phosphogee . AC Bonifacio 0:09.
electron affinity of phosphorus,Ago 11, 2023 Electron affinity of Phosphorus is 72 kJ/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is .
An atom of Phosphorus in the gas phase, for example, gives off energy when it gains an electron to form an ion of .To master the concept of electron affinity as a measure of the energy required to add an electron to an atom or ion. To recognize the inverse relationship of ionization energies .Learning Objectives. To understand the trends in properties and reactivity of the group 15 elements: the pnicogens. Like the group 14 elements, the lightest member of group 15, nitrogen, is found in nature as the free . Electron Affinity. Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to .Pelaez, R.J.; Blondel, C.; Vandevraye, M.; Drag, C.; Delsart, C., Photodetachment microscopy to an excited spectral term and the electron affinity of phosphorus, J. .

The electron affinity of phosphorus. DOI: 10.1088/0953-4075/40/20/010. Authors: P Andersson. Anton Oskar Lindahl. University of Gothenburg. C Alfredsson. L. . Electron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) . The electron affinity of group 4 elements is more than that of group 5 elements. This is because of the p orbitals of these elements. For example, phosphorous has its 3P orbital half-filled which is fairly stable so when one more electron is accepted the stability is disturbed. This is why the electron affinity of phosphorous is less than silicon.
Abstract. Black phosphorus (BP) is a two-dimensional (2D) layered semiconductor with tunable direct bandgap, high carrier mobility, balanced on/off ratio and significant crystal anisotropy. These properties provide unrivalled advantages for its applications in electronic and optoelectronic devices. To fulfill its potential, much .phosphorus. Formula: P. Molecular weight: 30.973762. IUPAC Standard InChI:InChI=1S/P Copy. IUPAC Standard InChIKey:OAICVXFJPJFONN-UHFFFAOYSA-N Copy. CAS Registry Number: 7723-14-0. Chemical structure: This structure is also available as a 2d Mol file. Other names: red phosphorus; Phosphorus atom. Permanent link for this .
The equations for second and higher electron affinities are analogous to those for second and higher ionization energies: E(g) +e− → E−(g) energy change=EA1 (7.5.4) E−(g) +e− → E2−(g) energy change=EA2 (7.5.5) As we have seen, the first electron affinity can be greater than or equal to zero or negative, depending on the electron . Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻(g), the .
The adiabatic electron affinities of five second row atoms (Al, Si, P, S, Cl) and their monoxides and dioxides were determined using six different density . Electron affinities of the oxides of aluminum, silicon, phosphorus, sulfur, and chlorine Nicole R. Brinkmann; Nicole R. Brinkmann Center for Computational Quantum Chemistry, .The table shows the first and second electron affinities for atoms of nitrogen and phosphorus. Why is the change in energy for both electron affinities lower for atoms of phosphorus than for atoms of nitrogen? [A] An atom of phosphorus has fewer electrons in its outer valence shell than an atom of nitrogen, and so there is less repulsion from the .
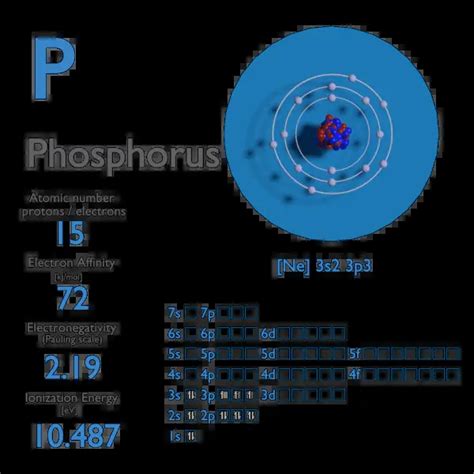
P: properties of free atoms. Phosphorus atoms have 15 electrons and the shell structure is 2.8.5. The ground state electron configuration of ground state gaseous neutral phosphorus is [ Ne ]. 3s2. 3p3 and the term symbol is 4S3/2. Schematic electronic configuration of phosphorus. The Kossel shell structure of phosphorus. We have measured the energies of all three fine structure components in the 3PJ ground state of the negative ion of phosphorus using laser photodetachment threshold spectroscopy. The experiment yielded an electron affinity of 746.68(6) meV. The ΔJ = 2–0, 2–1 and 1–0 fine structure splittings were determined to be 32.73(7) meV, . This electron pairing requires additional energy and thus it is easier to add electrons if there are free orbitals. When element has a half-filled p sublevel all 3 orbitals have one electron and pairing takes place (difference between energy levels of 2p and 3s is greater than electron pairing energy).electron affinity of phosphorus 7.5: Electron Affinities The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released .Phosphorus (symbol P) is a multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized . It has the highest electron affinity and the one of highest electronegativity of all the elements; thus chlorine is a strong oxidizing agent.
The electron affinity [EA] is the energy change for the process of adding an electron to a gaseous atom to form an anion (negative ion). X(g) +e− X−(g) EA1 (3.4.1) (3.4.1) X ( g) + e − X − ( g) EA 1. This process can be either endothermic or exothermic, depending on the element. The EA of some of the elements is given in Figure 3.4.6 3.4.
electron affinity of phosphorusElectron affinity can be defined in two equivalent ways. First, as the energy that is released by adding an electron to an isolated gaseous atom. The second (reverse) definition is that electron affinity is the energy required to remove an electron from a singly charged gaseous negative ion. . Phosphorus: 0.746 609(11) 72.037(1) 16 32 S .
phosphorus. Formula: P. Molecular weight: 30.973762. IUPAC Standard InChI:InChI=1S/P Copy. IUPAC Standard InChIKey:OAICVXFJPJFONN-UHFFFAOYSA-N Copy. CAS Registry Number: 7723-14-0. Chemical structure: This structure is also available as a 2d Mol file. Other names: red phosphorus; Phosphorus atom. Permanent link for this .
7.5: Electron Affinities Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .Electron Affinity: Electron affinity is a form of energy that is released during the addition of an electron to an element. The addition of electrons can make it a negative charge-bearing element. The electron affinity increases from left to right (in a period) and decreases down a column (in a group).In group 15, as elsewhere in the p block, we see large differences between the lightest element (N) and its congeners in size, ionization energy, electron affinity, and electronegativity (Table 22.3 "Selected Properties of the Group 15 Elements"). The chemical behavior of the elements can be summarized rather simply: nitrogen and .
electron affinity of phosphorus|7.5: Electron Affinities
PH0 · phosphorus
PH1 · The electron affinity of phosphorus
PH2 · Phosphorus
PH3 · Electron affinity (data page)
PH4 · Electron Affinity Chart (Labeled Periodic table + List)
PH5 · Electron Affinity
PH6 · 7.5: Electron Affinities
PH7 · 21.8 Nitrogen, Phosphorus, and the Group 5A Elements